Just say NO to viewed urine collection!

Toxicology testing that is easy for you and easy for your clients
This offers many benefits to both you and your clients, including:

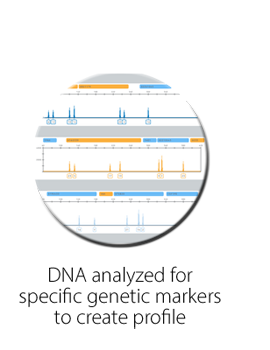
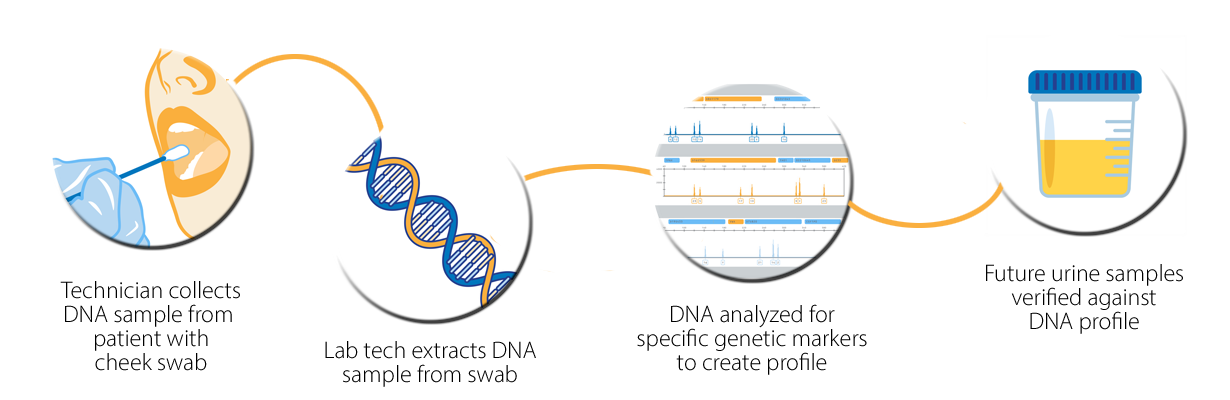
How does vTOX® DNA-verified urine drug testing work?


Comprehensive toxicology testing that helps you better serve your clients
Our testing requires only a small urine sample. Samples are verified by our vTOX® DNA-based technology so clients can't use artificial urine or urine from other sources.
Fast
Accurate
Convenient
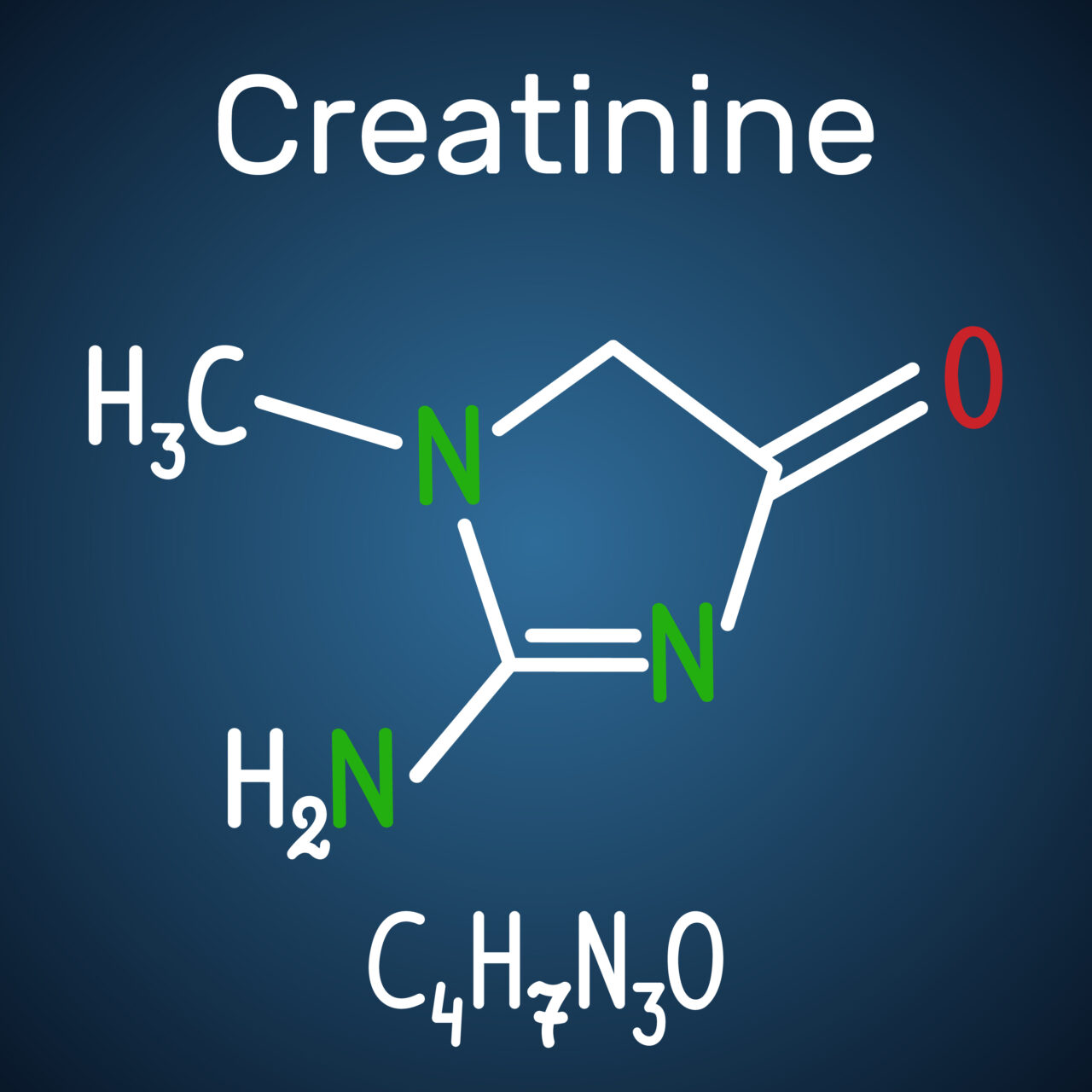
Standardizing to creatinine levels means more accurate results
By correlating creatinine levels with drug levels, our testing can determine if the urine sample has been diluted. This identifies any attempt by clients to artificially dilute their samples to mask drug use.
Standardizing the patient's results for the amount of creatinine present provides a more accurate assessment of substances present in the patient's body. Most reference labs do not standardize to creatinine levels, which can potentially lead to misleading results and missed treatment opportunities.
Fast and accurate toxicology testing for more than 50 substances

Unique partner portal makes it easy to enter orders and view test results
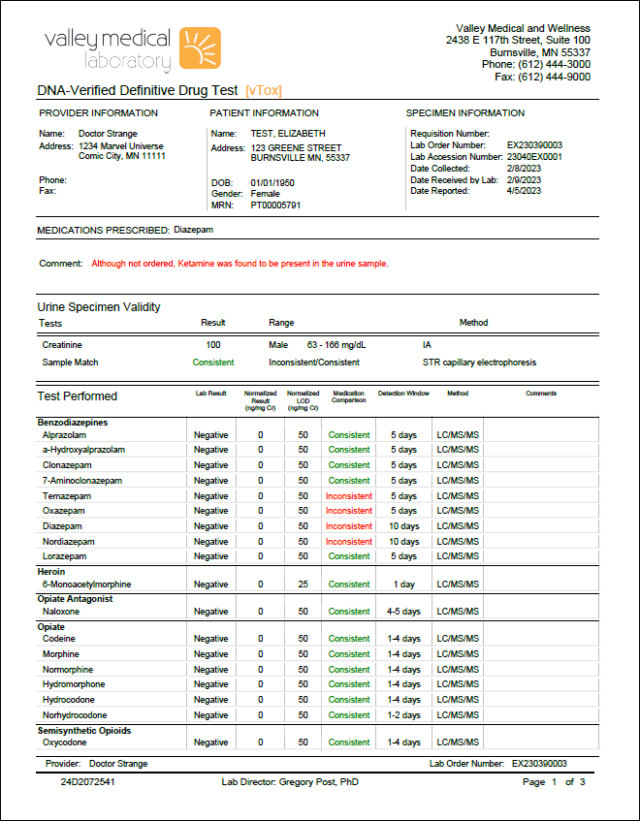
Comprehensive reporting helps you provide the right treatment
• Confirms DNA validation
• Provides detailed test results for more than 50 different analytes
• Identifies inconsistent results
It's everything you need to provide the right treatment for your clients.
Urine drug testing for
trauma-informed care
Urine drug testing for
telehealth addiction treatment
Urine drug testing that is
LGBTQ-friendly

Frequently Asked Questions
Valley Medical’s vTOX® DNA verification testing captures the size and presence of twelve specific genomic features referred to as short tandem repeats (STR). Due to inheritance and random events during replication, every individual has a unique size and frequency of these STRs. The test also measures the length of the Amelogenin gene, which is different between males and females.
Yes, the samples from patients who submit fake urine will either have no human DNA present or have non-matching DNA. If patients try to mix their sample with others, our test will detect an impossible number of alleles and the result will fail.
This test specifically and exclusively measures short tandem repeat (STR) regions that do not contain any relevant genetic risk factors or clinically relevant results. The results of this test can only be used for the matching of human samples.
No, the DNA samples we collect are safely disposed of after the initial analysis. We store only select DNA data, not the genetic material itself.
The genetic results of this test are compared to the patient’s reference sample profile. This validates the patient’s sex type as well as the twelve standard U.S. Core Loci to ensure sample identification.
Valley Medical’s vTOX® DNA verification is extremely accurate. The probability of a random match occurring are at most 1 in 150,000,000,000.
While a cheek swab is only necessary on the initial visit, vTOX® DNA-verification testing is conducted for every urine sample we receive. This ensures patient validity and eliminates the need for more invasive sample verification methods, such as viewed collections.
vTOX® DNA-verification testing takes the same amount of time as our standard toxicology analysis. Results will be reported within 36 hours of samples being received in our laboratory.

Is vTOX® right for your treatment center?
Contact us today to learn how vTOX® can benefit your treatment center facility!